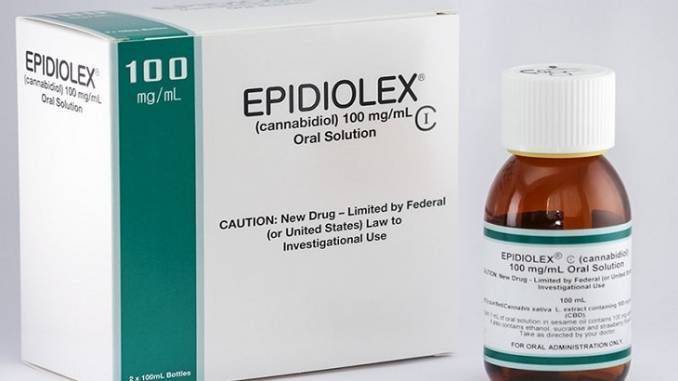
Cannabis-based epilepsy treatment Epidiolex will cost $32,500 annually
The expensive price tag means that the parents of children with Lennox syndrome and Dravet syndrome may search for cheaper CBD-based treatments elsewhere
Epilepsy sufferers may never have to worry about enduring bouts of life-threatening seizures ever again, thanks to the Food and Drug Administration (FDA) approving the world’s first ever cannabis-derived medication for the treatment of two rare types of pediatric epilepsy.
The discussion surrounding the cost of the medicine is stirring up a storm in the medical cannabis community. Investors have been informed of the average yearly cost and as it turns out, an annual treatment fee of $32,500 isn’t exactly feasible for most.
The cannabis-derived epilepsy treatment, known as Epidiolex, was developed by British company GW Pharmaceuticals. Head of GW’s commercialization efforts, Julian Gangolli, has explained how the price of the epilepsy treatment is necessary to ensure Epidiolex is on-par with the cost of alternative epilepsy drugs.
The estimated annual cost of Epidiolex was calculated as a result of obtaining insurance company feedback and those in need of the treatment aren’t exactly pleased with the news, to say the least.
FDA approves first drug comprised of active cannabis-derived ingredient
 In June, the U.S. Food and Drug Administration approved Epidiolex, an oral solution containing the non-psychoactive medicinal cannabinoid CBD (cannabidiol).
In June, the U.S. Food and Drug Administration approved Epidiolex, an oral solution containing the non-psychoactive medicinal cannabinoid CBD (cannabidiol).
The cannabis-derived treatment was developed to treat Lennox-Gastaut syndrome and Dravet syndrome, two severe forms of epilepsy, in young patients aged two years and above.
The breakthrough treatment was the first FDA-approved drug of its kind to consist of a purified cannabis-derived ingredient. No other treatment for Dravet syndrome has been approved by the FDA in the history of medicine.
Patients may have to wait up to three weeks to receive Epidiolex
 Not only will parents and/or guardians of young epileptic patients have to afford the hefty cost of Epidiolex every single year to treat their young but also, they must wait up to three weeks to receive treatment after obtaining a prescription from a healthcare practitioner or doctor.
Not only will parents and/or guardians of young epileptic patients have to afford the hefty cost of Epidiolex every single year to treat their young but also, they must wait up to three weeks to receive treatment after obtaining a prescription from a healthcare practitioner or doctor.
This is according to Gangolli, who has publicized the fact that the Drug Enforcement Agency (DEA) must first reschedule the active cannabis compound CBD before prescriptions can legally be written.
At the current time, CBD remains a Schedule 1 drug with no “accepted medical use.” After the FDA approved Epidiolex, the agency had a 90-day timeframe in which the drug could be reclassified or rescheduled as a schedule 2, 3, 4, or 5 substance.
“We don’t have a choice on that,” said the DEA public affairs officer Barbara Carreno during an interview with Business Insider back in June.
To this date, CBD has not been rescheduled or reclassified. However, this does not mean that the DEA won’t approve CBD for its medicinal benefits. When the milestone event occurs, doctors can legally prescribe Epidiolex to patients.
Even so, the question remains: How will patients be able to afford Epidiolex as an epilepsy treatment for their children?
There is a high probability that the parents of children with Lennox syndrome and Dravet syndrome will search for cheaper CBD-based treatments inside licensed and regulated medical cannabis dispensaries. Cannabis advocates and researchers are concerned about this. After all, how are the customers to know exactly what is contained in alternative CBD products that have not been FDA-approved?
Sure, dispensary shelves might be overloaded with CBD products, but the sources contained in each may not be as reputable as, let’s say, an FDA-approved epilepsy treatment like Epidiolex.
Insurance coverage could offer patients and parents with a window of opportunity, however. Gangolli affirms that, with insurance coverage in place, Epidiolex could be acquired for a fraction of the cost of dispensary-bought CBD products.
“The cost of a co-pay [for Epidiolex] is significantly – or could be significantly – less onerous and burdensome than the cost of the product either over the Internet or from a dispensary,” Gangolli said.