UCSD researchers will conduct study on cannabis to treat Essential Tremor (ET)
The double-blind, placebo-control, cross-over clinical trial will enroll 16 adult participants who have been diagnosed with ET by a movement disorder neurologist

Last week, UC San Diego researchers revealed their plans to carry out a cannabis trial that will determine the plant’s efficacy at treating essential tremor (ET).
It will kick off in the beginning of 2019 and will involve the use of an oral cannabis supplement with a CBD to THC ratio of 20:1.
The UCSD researchers are hopeful that their study findings will demonstrate the suitability of cannabis for ET.
“This study will provide key insights,” said UCSD neurologist Dr. Fatta Nahab. “If found to be safe and effective, cannabis would not only serve as an exciting new addition to the limited treatment options currently available for patients with ET, but it might also provide scientists with new insights on essential tremor.”
Cannabis for ET: What is Essential Tremor?
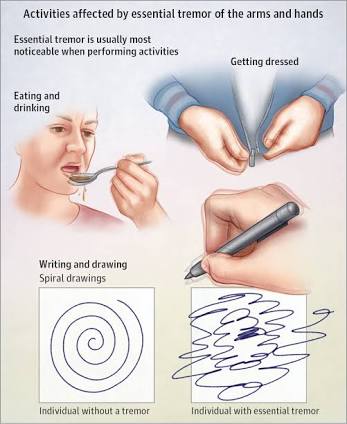
Patients with ET generally exhibit similar symptoms to those associated with Parkinson’s Disease (PD) however, the nervous system disorder being studied by UCSD researchers is eight times more common than PD.
According to Jane et al 2006, one-third of patients originally diagnosed with ET were in fact misdiagnosed, usually with PD.
Both conditions affect the body parts; primarily the hands. Someone who suffers from ET may endure kinetic tremor (occurs with voluntary movements,) intention tremor (occurs with goal-directed movement) and/or postural tremor (occurs when holding a position against gravity.)
Cannabis for ET: Weed may potentially replace traditional ET treatments
At the current time, ET is treated using medications that help stabilize high blood pressure and control seizures, such as beta blockers. These medications, despite being somewhat effective, have been known to carry certain side effects, ranging from heart palpitations to migraines.
Rather than focusing on cannabis’ psychoactive constituent, tetrahydrocannabinol (THC), the UCSD researchers will explore the therapeutic properties of its non-psychoactive cousin, cannabidiol (CBD).
“The double-blind, placebo-control, cross-over clinical trial will enroll 16 adult participants who have been diagnosed with ET by a movement disorder neurologist. All study participants will be gradually administered an oral cannabis formulation with a 20:1 ratio of CBD to THC. After completing a two-week period at the maximum target dose, participants will taper off, followed by a washout period before crossing over to the alternate study arm,” the study’s press release states.
Cannabis for ET: Double-blind study will involve the use of placebos

The “placebo effect” is a debatable topic. For the UCSD researchers leading this study on cannabis for ET, it plays an important role in ascertaining what works and what doesn’t for patients with rhythmic shaking caused by neurological disorders.
In order to orchestrate the study, researchers will employ 16 adults who have been diagnosed with ET. The double-blind study will see half of the study subjects getting administered with a placebo, while the other half is treated using cannabis.
The UC San Diego School of Medicine’s Center for Medicinal Cannabis Research will team up with UCSD researchers to oversee this study on cannabis for ET. Based on Nahab’s predictions, the researchers will have accumulated satisfactory results by the year 2020.
“This work expands CMCR’s commitment to develop an evidence-based approach in the area of cannabinoid therapeutics,” said the director of the Center for Medicinal Cannabis Research, Dr. Igor Grant. “The oral combination of CBD and THC is the first-of-its-kind to be studied and is especially interesting to CMCR.”
Phase one and two of the clinical trial will be the first to investigate the potential of using cannabis to treat ET. The study will run for approximately one year.